The term “Nutrigenomics” refers to the study of how the structure of our genes influences our interaction with foods and specific nutrients. In other words, the foods we eat may exert different influences upon each of us, based on the unique structure of the DNA in each individual. Nutrigenomics, as a science, is continuously evolving based on our understanding of what we call “gene-nutrient interactions” (how nutrients can influence our genes), as well as “gene-gene interactions” (how one gene can influence the activity of another gene). Historically, Nutrigenomics was conceptualized around the idea that the genetic sequence of our DNA is the most important factor in establishing who we are from a biologic standpoint. That is, with the advent of the Human Genome Project, most scientists believed that, once we know our DNA sequence, we could predict most of the health outcomes, or the risk for various diseases. This was happening in the first decade of the 21st century.
However, we soon came to the realization that, when we look at the risk of chronic metabolic diseases (such as obesity, type 2 diabetes, metabolic syndrome, etc.), and their associated conditions (such as the risk of many cardiovascular diseases), the DNA sequence of a given gene can explain only in part our propensity for such conditions. When looking at one gene at a time, we typically see that such genetic variation can explain the risk of disease in the magnitude (usually) of under 10%. This means that a certain genetic variation (a certain DNA sequence) is responsible only for around 10% of disease cases.
This led to a deeper understanding of what genetic variations mean for our health. One idea is that such metabolic diseases could be the result of genetic variations in more than one gene. This is the concept of “polygenic traits“: more genes work together towards establishing a biological outcome. The more genes are affected, the more chances one has to develop a certain condition or disease. [Certainly, there are few cases of monogenic nutrition-related diseases such as Phenylketonuria (PKU), but these are not the subject of our presentation. Such monogenic diseases are well understood today, and we have efficient interventions at hand]. Besides the concept of polygenic diseases (or traits), another concept developed, which refers to “gene-environment interactions“. It became quickly obvious (using epidemiological studies in different populations), that the risk of metabolic disorders was also influenced by environmental factors. In other words, people having the same genetic variations may have different risk of disease if they live in different environments. Maybe the best and oldest example is the study of Japanese people who emigrated to US. As compared with those from Japan or Hawaii, the Japanese emigrants to California had an increased risk of disease, which could be explained by the change in their environment, including nutrition (read more here).
Meanwhile, another story was developing beginning with 1940s. As opposed to the idea that variations in DNA sequence were responsible for most health outcomes, the proponents of “Epigenetics” contended that there are other ways that acquired traits can be passed on to the next generation, rather than only the inheritance of DNA sequences. Most of scientists paid little attention to this theory, until 1980s, when technological advances made possible to objectivise this concept reliably. It turned out that our DNA is not only a passive container of genetic information (our DNA code), but it can also be chemically modified without changing its DNA sequence. This process is called “DNA methylation“, in which methyl groups (CH3) are attached to DNA nucleotides. Several amazing discoveries made us understand that:
– DNA methylation can regulate how genes work;
– The DNA methylation of some genes can be inherited by our children;
– The DNA methylation status of a gene can be changed by what we eat;
– Therefore, it is possible that changes in our DNA methylation status to be passed on to our children.
This is how the adage “We are what we eat” came in the top-chart of nutrition slang.
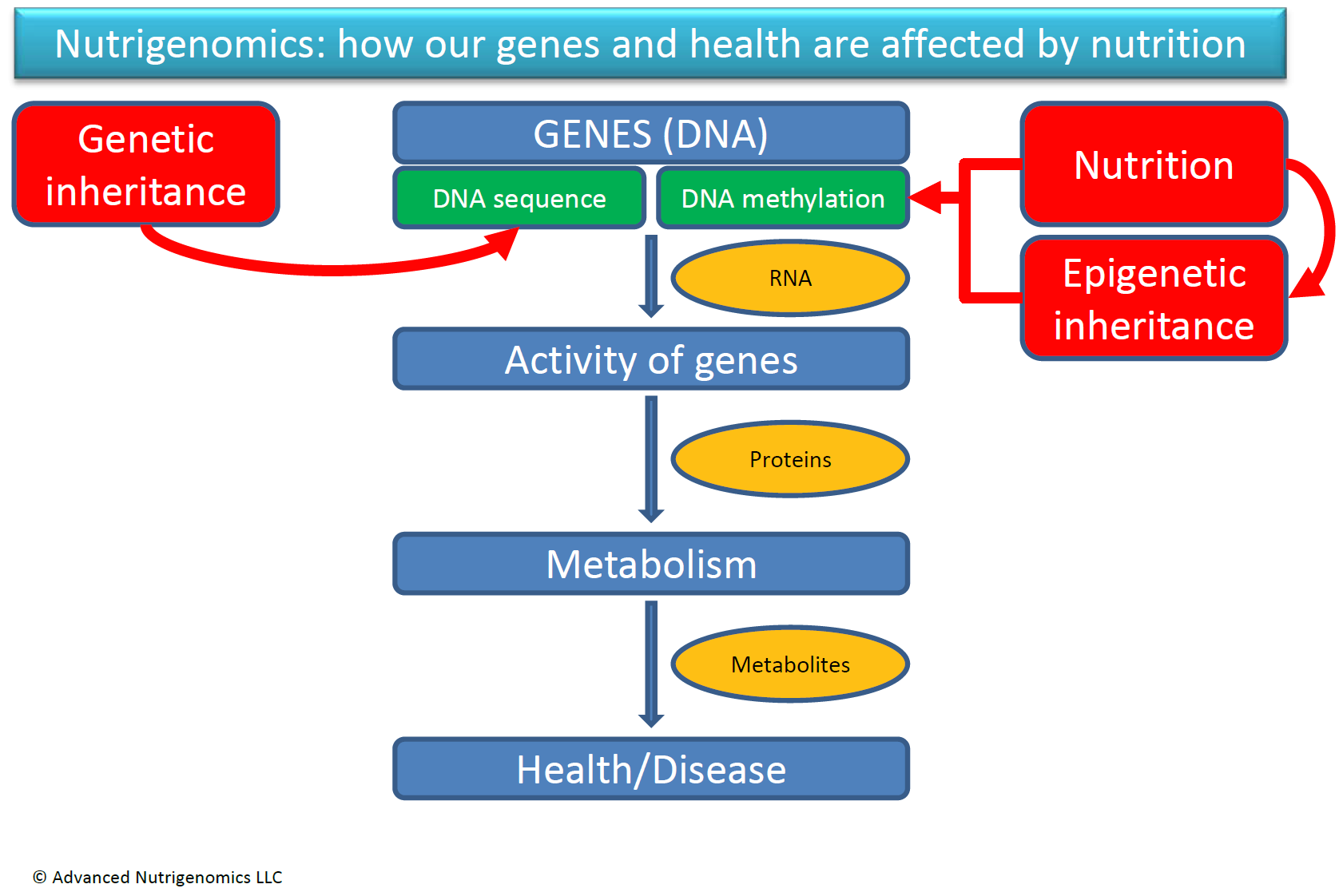
In conclusion, we know today that the way we respond to nutrition is a compounded result of both our genetic sequence (genetics) and the chemical modifications (i.e. DNA methylation) that occur as result of nutrition and other environmental factors (epigenetics). The figure on this page depicts our current understanding of gene-nutrient interactions.
